The Periodic Table - From Classification to Law, from Law to System and finally from System to Table
From Classification to Law, from Law to System and finally from System to Table
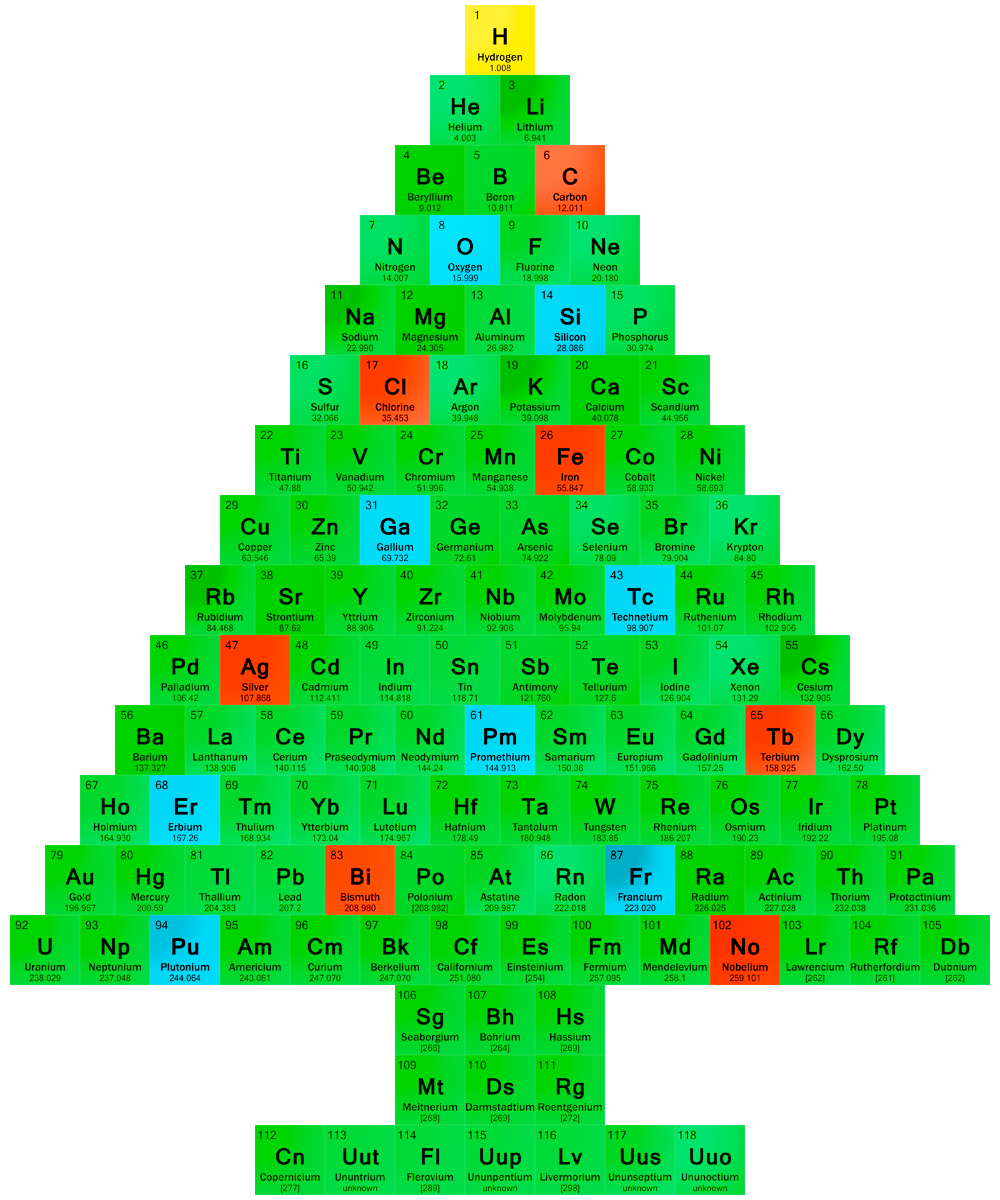
The Periodic Table (System) was discovered in an era when atomic structures and electrons where not known, and equipment to purify and separate elements was still primitive. The discoveries of Mendeleiev, Meyer and others are therefore to be seen as immense. After the first International Conference of Chemists in 1860 (Karlsruhe), which both Mendeleiev and Meyer attended, it became clear that a number of scientists had noted some regularities between chemical elements. The discoveries published in 1869 by Mendeleiev, first in a vertical order, later that year in a horizontal arrangement, were preceded by discoveries of similar "regularities" from Béguyer de Chancourtois, Newlands, Odling, Hinrichs and Lothar Meyer. Only Meyer produced a quite similar tabular arrangement, in fact just after Mendeleiev. There is little discussion that Mendeleiev published his system noting that there was a periodic classification, i.e. the periodic law and the systematic arrangements of the elements, including some of the not yet discovered elements for which he even predicted chemical properties. Despite the fact that some of these predictions were incorrect and that in his system there was no place for the Noble Gases, he is still generally accepted as the chief architect, since he discovered the "system"; only later it was changed to "Table" as we now use in Periodic Table of Chemical Elements. Remarkable, the word "System" is still used as in "Periodic System" in a number of languages, e.g. Danish ("Periodiske system"), Dutch ("Periodiek systeem") and German ("Periodensystem"), just as Mendeleiev and Meyer used it in their papers.
Back to The Periodic Table
[Text and images from IUPAC.]