The Periodic Table - History of discovery
History of discovery
The International Year of the Periodic Table of Chemical Elements in 2019 will commemorate a remarkable series of important milestones in the history of the Periodic Table of Chemical Elements dating back 2800, 350, 230, 190, 150, and 80 years.
Video "300 years of element discovery" here.
Video "Tudo se Transforma - História da Tabela Periódica" for general public here.
Around 800 BC, an Arab alchemist named Jabir ibn Hayyan first isolated the chemical elements arsenic and antimony. In 1669, phosphorus was the first element to be chemically discovered by Hennig Brandt (German). In 1789, Antoine Lavoisier (French) published a list of 33 chemical elements grouped into gases, metals, nonmetals, and earths. In 1829, Johann Wolfgang Döbereiner (German) observed that when many of the elements were grouped in three (triads) based on their chemical properties and arranged by atomic weight, the second member of each triad was approximately the average of the first and the third (Law of Triads). In 1869, Dmitri Mendeleiev (Russian) developed the modern periodic table as it is known today. In 1939, a French woman scientist, Marguerite Perey, discovered element francium based on filling gaps in Mendeleiev’s Periodic Table. It is also believed that lead smelting began at least 9,000 years ago in Africa, and the oldest known artifact of lead is a statuette found at the temple of Osiris on the site of Abydos (Egypt) dated circa 3800 BC.


Dmitri Mendeleiev
March 1, 1869 is considered as the date of the discovery of the Periodic Law. That day Dmitri Mendeleiev completed his work on ''The experience of a system of elements based on their atomic weight and chemical similarity''. This event was preceded by a huge body of work by the most outstanding chemists in the world.
By the middle of the 19th century, 63 chemical elements were already discovered, and attempts to find regularities in this set had been made repeatedly. In 1829, Döbereiner published the "Law of Triads": the atomic mass of many elements is close to the arithmetic mean of two other elements close to the original one in chemical properties (strontium, calcium and barium; chlorine, bromine and iodine, etc.).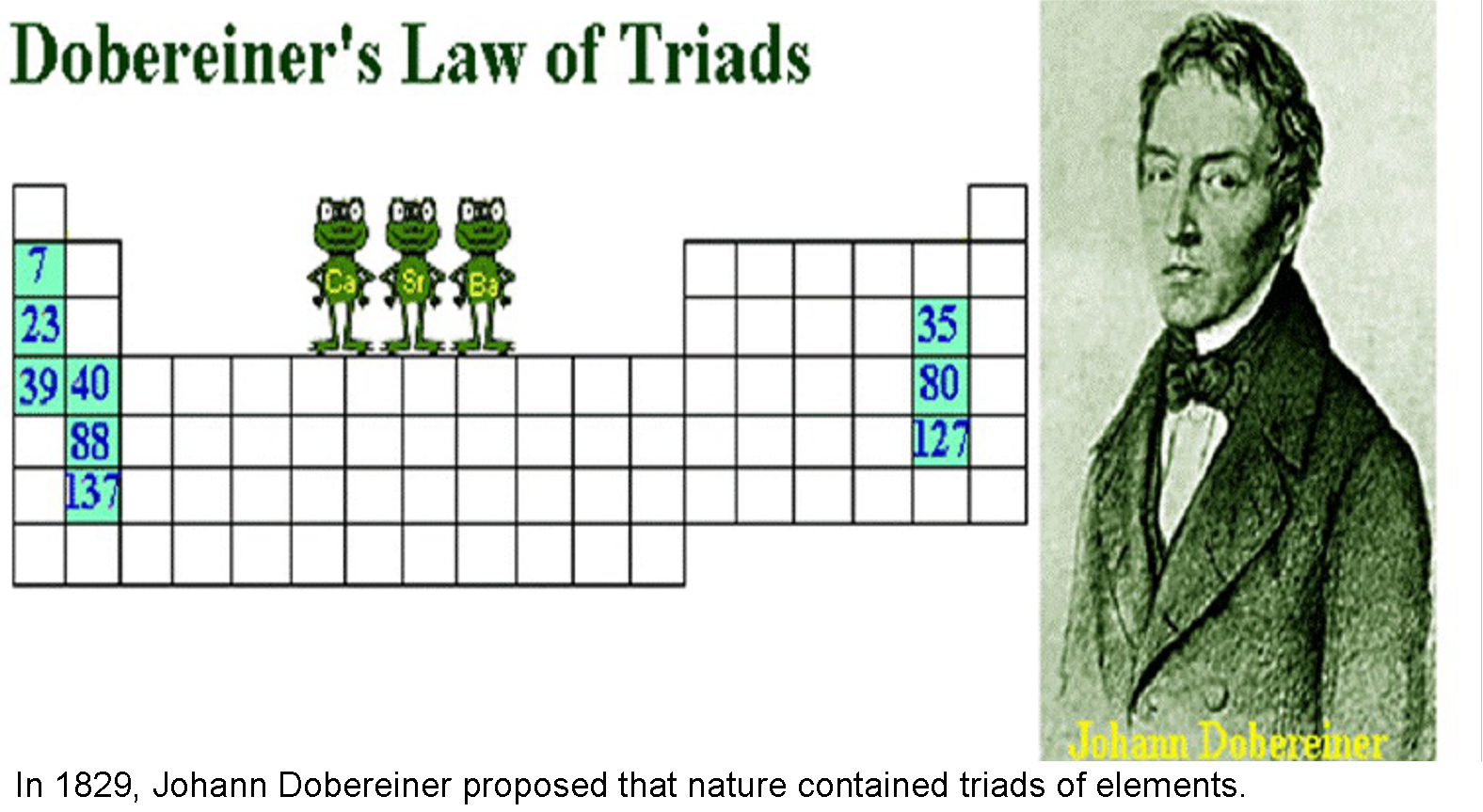
[Image from Julia Cheng]
The first attempt to arrange the elements in order of increasing atomic weights was undertaken by Alexandre-Émile Béguyer de Chancourtois (1862), who placed the elements along the helix and noted the frequent cyclic recurrence of their chemical properties along the vertical axis.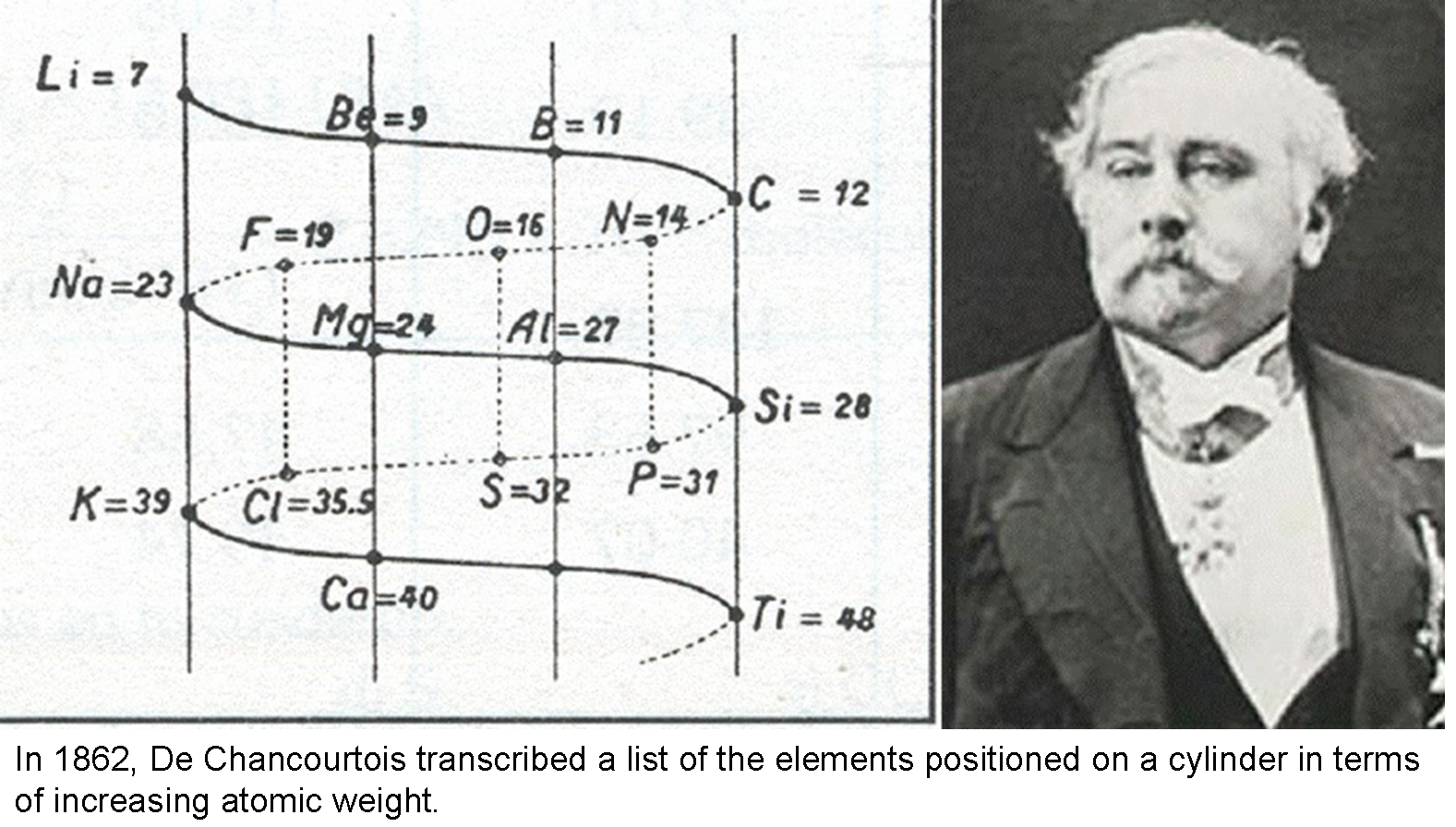
[Image from Julia Cheng]
Neither of these models attracted the attention of the scientific community. In 1866, chemist and musician John Alexander Reina Newlands suggested his version of the periodic system "Law of Octaves" looked a bit like Mendeleiev’s one. However, it was compromised by the author’s persistent attempts to find mystical musical harmony in the table. In the same decade, several more attempts were made to systematize chemical elements. 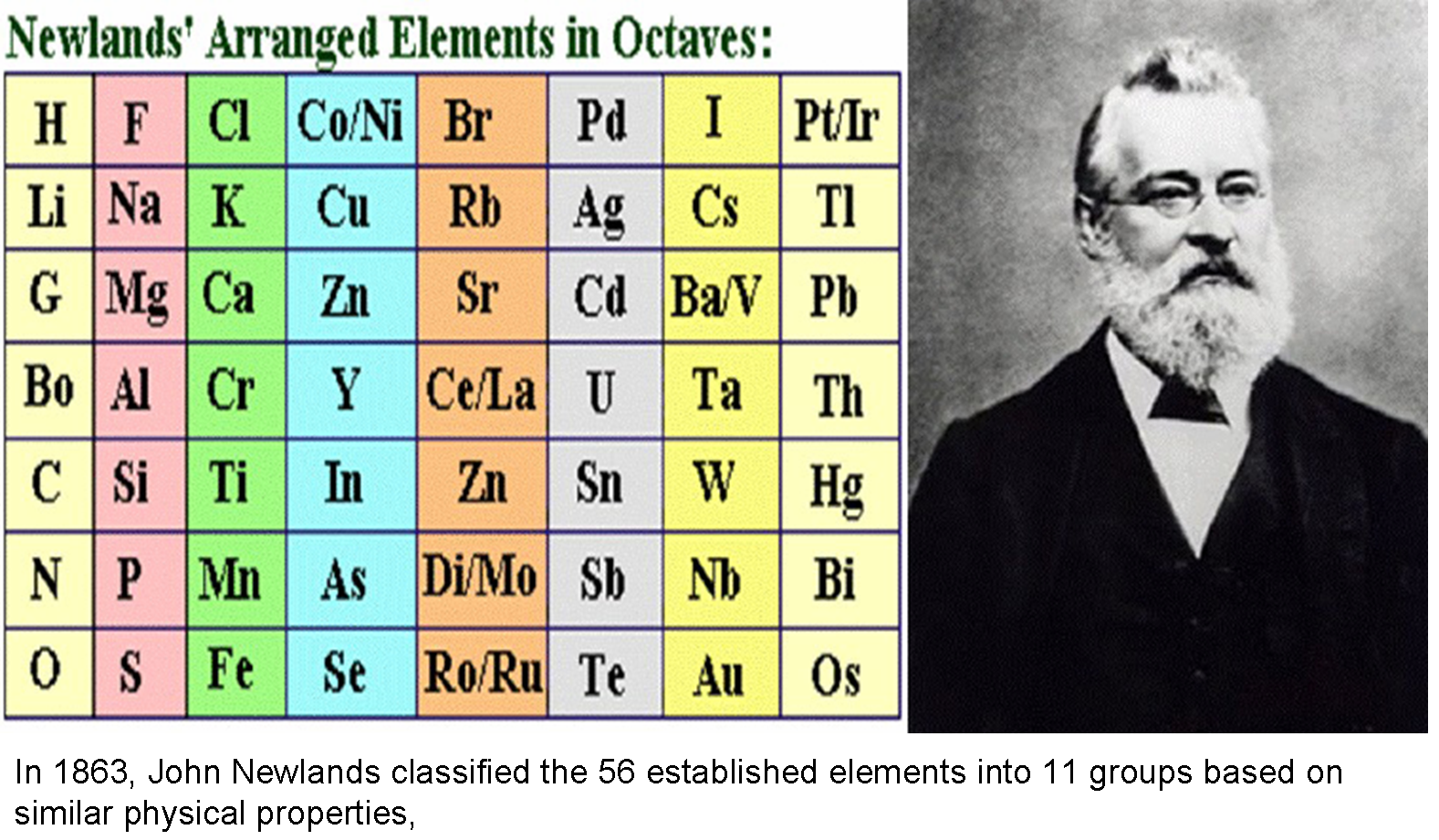
[Image from Julia Cheng]
Julius Lothar Meyer was very close to the final version (1864). He published a table containing 28 of the 56 know elements using valency as the basis for periodicity. Dmitri Mendeleiev published his first diagram of the periodic table in 1869 in the article "The Correlation of Properties with the Atomic Weight of Elements" (in the Journal of the Russian Chemical Society). A bit earlier he sent a scientific announcement of the discovery to leading chemists of the world. This table included all the 61 known elements and allowed chemical properties/valency to dominate over atomic weight. He challenged some of the know atomic weights and predicted that there were certain elements still to be discovered.
As has been mentioned, March 1, 1869 is considered as the day of the discovery of the Periodic Law. That day Dmitri Mendeleiev completed his work on "The experience of a system of elements based on their atomic weight and chemical similarity". Meyer published an updated version of his table, which was very similar to that of Mendeleiev, in December, 1869.
In the early days, both Mendeleiev and Meyer were honored for their discovery of the "periodic relations of the atomic weights", sharing the Davy Medal of the Royal Society in 1882. Nowadays, Mendeleiev is almost universally accepted as the originator of the Periodic Table of the Chemical Elements, perhaps because he included all known elements and because he used the Table predictively. Subsequently, the unknown elements he had predicted, gallium (1875), scandium (1879) and germanium (1887) were discovered and had the properties he predicted for them. 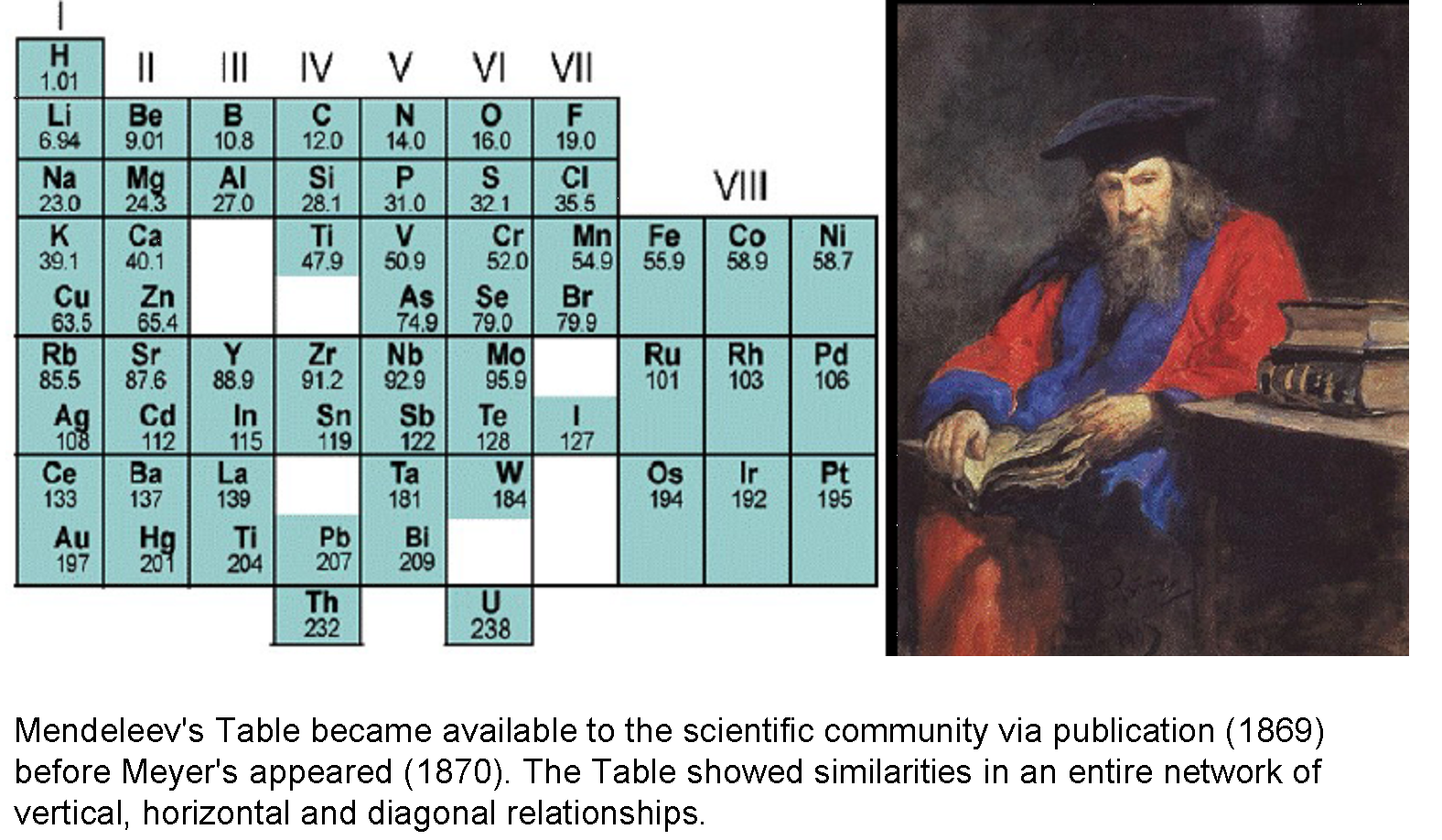
[Image from Julia Cheng]
According to legend, the idea of a system of chemical elements came to Mendeleiev in a dream, but it is known that when asked how he discovered the periodic system, the scientist replied: "I've thought about it for twenty years, but you think: he was sitting and suddenly ... it's ready".
Back to The Periodic Table
[Text from IUPAC.]