The Periodic Table - New Elements
Discovery of new elements of the Periodic Table
After the discovery in 1940-41 of the first artificial elements – neptunium and plutonium – the problem of the boundaries of the Periodic Table, the nuclear and chemical properties of the extremely heavy nuclei turned out to be of fundamental interest for natural scientists. Studies have now been carried out for many years in the major research nuclear centers in Germany, the USA, Japan, France, China and in the Flerov Laboratory of nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia.
By the beginning of the 1950 all available cells in the Periodic table of chemical elements had been filled and 8 transuranic elements have been synthesized. New elements were synthesized by the successive capture of neutrons by uranium nuclei in nuclear reactors, or by prompt capture of 15 – 20 neutrons in thermonuclear explosions.
A fundamentally new approach to the synthesis of elements in fusion reactions of heavy nuclei was proposed independently by professors A. Ghiorso and G. Seaborg at the Berkeley National Laboratory, USA and by professor G. Flerov in the Laboratory No. 2 of the Academy of Sciences in Moscow, USSR (now it is the National Research Center "Kurchatov Institute").
On the initiative of Prof. G. Flerov the Laboratory of Nuclear reactions was founded in the Joint Institute for Nuclear Research (JINR), organized at Dubna near Moscow in 1956. The new laboratory was equipped with a heavy-ion accelerator U300. This was the start of a new direction in nuclear physics – heavy ion physics.
The International Unions of Pure and Applied Physics (IUPAP) and Chemistry (IUPAC) recognized the priority of Dubna in the discovery of elements 102-105 and marked the great contribution of JINR in the discovery of elements 106-108. In 1997 at the general Assembly of IUPAC element No 105 was named "Dubnium" as a sign of recognition of the key role of the Laboratory of Nuclear Reactions in the outlining of the scientific strategy and synthesis of superheavy elements. Like berkelium and dubnium, the element 110 darmstadtium honors the city of Darmstadt (Germany) which houses the Helmholtz Centre for Heavy Ion Research GSI where 6 new elements (107 – 112) had been discovered.
By the end of the last century, 20 artificial transuranic elements had been discovered. It was found that the nuclear stability and the probability of formation of transuranic elements decrease dramatically with increasing atomic number. It was thought that even a modest advance into the region of even heavier elements would lead to the limit of their existence.
By the end of the 1990s, the scientists in the Dubna Laboratory of nuclear reactions managed to make a break-through in the synthesis of superheavy elements and in the understanding of the problem of their stability. Due to the achieved high efficiency of heavy ion beams acceleration and the considerable improvement of the experimental methods, the new elements with atomic numbers 113 - 118 were synthesized for the first time.
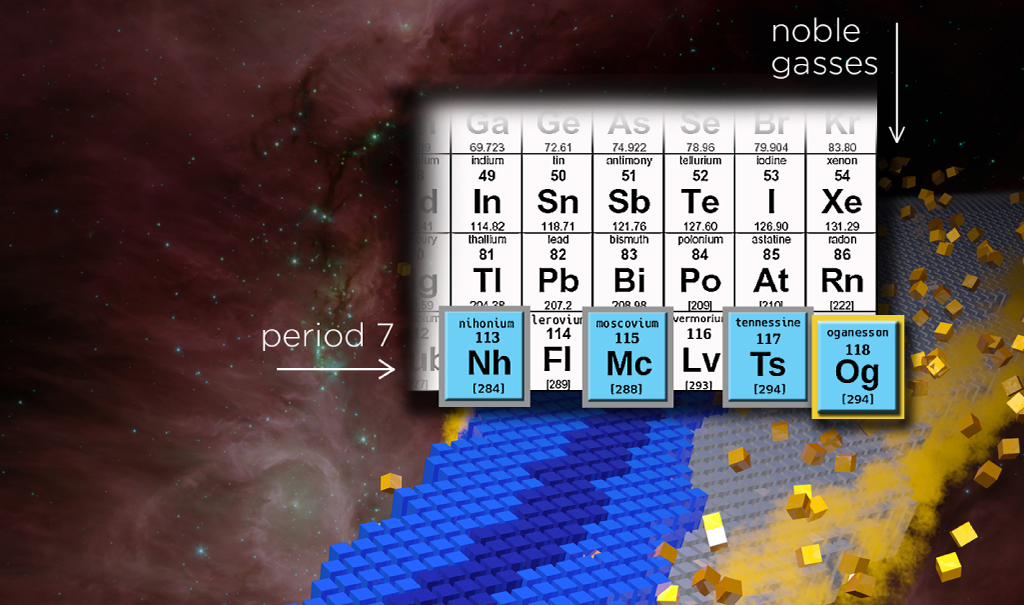
For the element with atomic number 113 the discoverers at RIKEN Nishina Center for Accelerator-Based Science (Japan) proposed the name nihonium and the symbol Nh. Nihon is one of the two ways to say “Japan” in Japanese, and literally mean "the Land of Rising Sun".
For the element with atomic number 114 the discoverers proposed the name flerovium and the symbol Fl. For the element with atomic number 115 the name proposed was moscovium with the symbol Mc. These names are in line with tradition of honoring a place or geographical region and are proposed jointly by the discoverers at the Joint Institute for Nuclear Research, Dubna (Russia), Oak Ridge National Laboratory (USA), Vanderbilt University (USA) and Lawrence Livermore National Laboratory (USA). For the element with atomic number 116 the name proposed is livermorium with the symbol Lv. This is again in line with tradition and honors the Lawrence Livermore National Laboratory (1952).
For the element with atomic number 117 the name tennessine with the symbol Ts was accepted. Tennessine is in recognition of the contribution of the Tennessee region, including Oak Ridge National Laboratory, Vanderbilt University, and the University of Tennessee at Knoxville, to superheavy element research, including the production and chemical separation of unique actinide target materials for superheavy element synthesis at ORNL’s High Flux Isotope Reactor (HFIR) and Radiochemical Engineering Development Center (REDC).
For the element with atomic number 118 the collaborating teams of discoverers at the Joint Institute for Nuclear Research, Dubna (Russia) and Lawrence Livermore National Laboratory (USA) proposed the name oganesson and symbol Og. The proposal is in line with the tradition of honoring a scientist and recognizes Professor Yuri Oganessian for his pioneering contributions to transactinoid elements research. His many achievements include the discovery of superheavy elements and significant advances in the nuclear physics of superheavy nuclei including experimental evidence for the "island of stability".
The discovery of element 118 completes the 7th row of the periodic table. Scientists accociate the further progress in the synthesis of the 8th row elements with the creation in Dubna of the modern accelerator complex - the world’s first factory of superheavy elements. The question of the boundaries of the Periodic table of elements remains open.
Back to The Periodic Table
[Text and images from IUPAC.]